digene HC2 High-Risk HPV DNA Test
For detection of human papillomavirus infections using Hybrid Capture 2 technology
For detection of human papillomavirus infections using Hybrid Capture 2 technology

Cat. No. / ID: 5199-1220
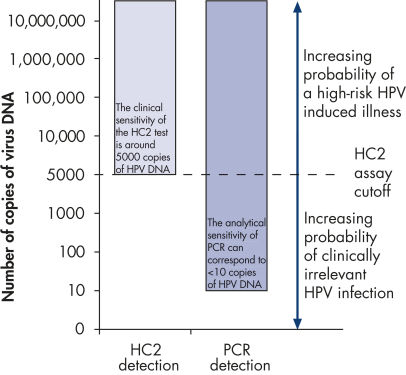
The digene HC2 High-Risk HPV DNA Test is FDA-approved for in vitro diagnostic use (see IVD mark). Using Hybrid Capture 2 technology for HPV testing offers the following benefits when compared with PCR for HPV testing:
The digene HC2 High-Risk HPV DNA Test detects the presence of 13 high-risk HPV types (16/18/31/33/35/39/45/51/52/56/58/59/68, which are carcinogenic) using full genome probes complementary to HPV DNA, specific antibodies, signal amplification, and chemiluminescent detection. It analyzes HPV DNA high-risk groups in cervical specimens.
The digene HC2 High-Risk HPV DNA Test has been approved for use with the digene HC2 DNA Collection Device, digene HC2 Sample Conversion Kit, digene Cervical Sampler, digene Specimen Transport Medium, and PreservCyt Solution (Hologic), depending on country regulations. Automated options for the digene HC2 High-Risk HPV DNA Test include the Rapid Capture System (for high-volume sample throughput testing); guidelines vary among countries.
The digene HC2 High-Risk HPV DNA Test uses advanced, Hybrid Capture 2 technology to directly detect HPV. It is the most widely accepted HPV test, providing extensive validation in conjunction with Pap in clinical studies.
The digene HC2 High-Risk HPV DNA Test detects the presence of 13 high-risk types using full genome RNA probes complementary to the HPV DNA, specific antibodies, and chemiluminescent detection. The target DNA combines with specific RNA probes, creating RNA:DNA hybrids. Then, the RNA:DNA hybrids are captured onto a solid phase coated with universal capture antibodies specific for RNA:DNA hybrids. The specimen matrix is then washed from the captured hybrids to remove inhibitors. During the signal amplification, captured RNA:DNA hybrids are detected with multiple antibodies conjugated to alkaline phosphatase. The signal resulting from the chemiluminescent reaction is read and the results are automatically interpreted.
The digene HC2 High-Risk HPV DNA Test is used to screen patients with ASC-US (atypical squamous cells of undetermined significance) Pap results to determine the need for referral to colposcopy. The results of this test are not intended to prevent women from proceeding to colposcopy.
In women 30 years of age and older, the digene HC2 High-Risk HPV DNA Test can be used with Pap to adjunctively screen to assess the presence or absence of high-risk HPV types. Guidelines for use of the HPV Test vary between countries, but in countries such as the USA, experts recommend that women 30 years of age and older get the HPV Test along with the Pap test. The presence or absence of high-risk HPV types, together with the physician's risk assessment of cytology history, other risk factors, and professional guidelines, may be used to guide patient management.