QIAsymphony DSP Virus/Pathogen Kit
For fully automated purification of viral nucleic acids or bacterial DNA
For fully automated purification of viral nucleic acids or bacterial DNA
QIAsymphony DSP Virus/Pathogen Kits, in combination with the QIAsymphony SP, enable automated purification of viral nucleic acids and bacterial DNA from a broad range of sample materials for in vitro diagnostic use. Kits are available in mini and midi format for sample volumes of 200 μl and up to 1000 μl. Optimized protocols provide high yields of pure nucleic acids, and flexible elution volumes allow nucleic acid concentration to be easily optimized for each downstream application. Novel, prefilled reagent cartridges reduce setup time and minimize manual handling. Bar code reading enables full tracking of reagents.
QIAsymphony DSP Virus/Pathogen Kits can be used to purify viral nucleic acids from serum, plasma, or CSF, and viral nucleic acids and bacterial DNA can be purified from respiratory samples such as swabs, as well as urine and urogenital swabs (cervical and urethral). The kits can be used to purify nucleic acids from a broad range of DNA and RNA viruses, as well as bacterial DNA from Gram-negative and Gram-positive bacteria.
QIAsymphony DSP Virus/Pathogen Kits combine the speed and efficiency of silica-based purification of viral and bacterial nucleic acids with the convenient handling of magnetic particles (see " Schematic of the QIAsymphony SP principle"). Innovative, ready-to-run reagent cartridges are prefilled with all reagents required for the purification procedure and are automatically opened by the QIAsymphony SP, which helps to minimize the risk of environmental contamination (see figure " Prefilled, sealed reagent cartridges"). Optimized protocols provide high quality nucleic acids with efficient removal of proteins, nucleases, and other impurities. Nucleic acids purified with QIAsymphony DSP Virus/Pathogen Kits are ready for direct use in downstream applications (e.g., PCR). The user can choose between different elution volumes.
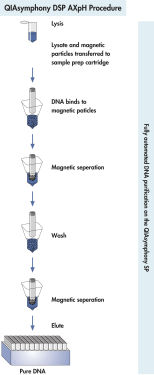
The purification procedure is designed to ensure safe and reproducible handling of potentially infectious samples, and comprises 4 steps: lyse, bind, wash, and elute (see " QIAsymphony DSP Virus/Pathogen procedure"). Worktable setup is rapid, which saves your valuable time. Simply place up to 2 reagent cartridges in the consumables drawer. The reagent cartridges can be either from the same kit or from different kits, enabling different purification procedures to be performed within the same run of 96 samples. Reagent volumes only for the selected number of samples are used, giving you complete cost control.
| Protocol | Kit | Sample material | Volume processed |
|---|---|---|---|
| Cellfree 200 | QIAsymphony DSP Virus/Pathogen Mini Kit | Plasma, serum, and CSF | 200 µl |
| Cellfree 500 | QIAsymphony DSP Virus/Pathogen Midi Kit | Plasma, serum, and CSF | 500 µl |
| Cellfree 1000 | QIAsymphony DSP Virus/Pathogen Midi Kit | Plasma, serum, and CSF | 1000 µl |
| Complex 200 | QIAsymphony DSP Virus/Pathogen Mini Kit | Respiratory samples (dried swabs, transport media) and urogenital samples (urine, transport media) | 200 µl |
| Complex 400 | QIAsymphony DSP Virus/Pathogen Midi Kit | Respiratory samples (dried swabs, transport media) and urogenital samples (urine, transport media) | 400 µl |
| Complex 800 | QIAsymphony DSP Virus/Pathogen Midi Kit | Respiratory samples (dried swabs, transport media) and urogenital samples (urine, transport media) | 800 µl |
QIAsymphony DSP Virus/Pathogen Kits enable sensitive detection of a broad range of DNA and RNA viruses, as well as bacterial DNA. Yields are linear, allowing accurate quantitative analysis for both low and high viral or bacterial titers. The high-quality nucleic acids are ready to use in all downstream applications, including sensitive detection assays using quantitative, real-time PCR or RT-PCR.